Product Categories
Products
-
 Plasmid Midiprep Columns
$74.99 – $89.99
Plasmid Midiprep Columns
$74.99 – $89.99
-
 0.2 ml PCR Tubes with Attached Caps
$99.99
0.2 ml PCR Tubes with Attached Caps
$99.99
-
 Immunoaffinity Chromatography Kits
Immunoaffinity Chromatography Kits
-
 10 μl (0.1 - 10 μl) LongReach Tips
$64.99
10 μl (0.1 - 10 μl) LongReach Tips
$64.99
-
 Multi-Drugs Screen Test -(FDA 510(k) Approved)
$5.99 – $269.99
Multi-Drugs Screen Test -(FDA 510(k) Approved)
$5.99 – $269.99
Filter by Price
Desalting Spin Columns
$99.00
INTENDED USE
CommaX TM G-25 desalting spin columns works with high-speed centrifuges to process large samples in parallel in short time. They are very affordable and handy tools for protein desalting. Quickly desalt protein and biological samples for manual or automated liquid chromatography (LC) with BeingBio Spin Desalting columns, which offers excellent protein desalting and recovery in a centrifuge format.
Each desalting spin column includes: one collection tube, one adapter, one 12-mL prepacked chromatographic column, one upper cap, one bottom cap and two frits.
FEATURES
- Large sample volumes
- High desalting efficiency and recovery
- Timesaving
Introducing BeingBio’s Premier Dry Chemistry Analyzer – a revolutionary leap in clinical chemistry testing. This cutting-edge device is designed for rapid, accurate, and easy-to-use analysis, ensuring you get reliable results with minimal effort. Employing dry chemistry technology, it eliminates the need for calibration and water, simplifying preparation and maintenance. Capable of analyzing a wide range of clinical chemistry parameters from just a small sample volume, it’s perfect for fast-paced medical environments.
The analyzer boasts an intuitive operation, delivering results in as little as 12 minutes through a straightforward three-step process. It’s equipped with a comprehensive quality control system, ensuring the accuracy of every analysis. Designed for versatility, it can process whole blood, serum, or plasma, making it suitable for a broad spectrum of diagnostic applications.
Compact and portable, it’s an ideal solution for on-site testing, emergency diagnostics, and primary health care settings. Its automated features and smart connectivity options streamline workflow, enhancing laboratory productivity and data management efficiency.
INTENDED FOR: (But not limited to the list)
- Blood Fat Combo Tests:
Triglycerides(TG),
Total Cholesterol (TCHO),
High-Density Liptein Cholesterol (HDL-C),
Low-Density Lipoprotein Cholesterol (LDL-C) - Liver Function Combo Tests:
Alanine Aminotransferase(ALT),
Aspartate Aminotransferase(AST),
Albumin(ALB),
Total Bilirubin(TBIL) - Diabetic Hyperglycemia:
Urine Creatinine(UCR),
Urine Microalbumin(UMA),
Glucose(GLU)/Alanine Aminotransferase(ALT)/Triglycerides(TG)/Total Cholesterol(TC) - Renal Function Combo Tests:
Carbamide(Urea),
Creatinine(Cre),
Uric Acid(UA) - Gardiouacalar Disease:
Glucose(GLU),
Creatine Kinase Isoenzyme(CK-MB),
Lactate Dehydrogenase(LDH)
Ecstasy (MDMA) Drugs Test – [FDA 510(k) Approved]
$59.99 – $109.99


INTENDED USE
The Drug of Abuse test is a lateral flow chromatographic immunoassay for the qualitative detection of multiple drugs and drug metabolites in urine at specified cut-off level of the following drugs:
— Ecstasy (MDMA)
TEST PROCEDURE:
Review “Specimen collection ” instructions. Test devices, patient’s samples, and controls should be brought to temperature (20-30℃) prior to testing. Do not open pouches until ready to perform the assay.
2.Hold the dropper vertically and transfer 3 full drops of urine to the specimen well(S) of the test cassette, and then start the timer. Use a separate pipette and cassette for each sample or control.
3.Read result between 3 to 8 minutes after the addition of samples. Do not read result after 10 minutes.
PACK SIZE:
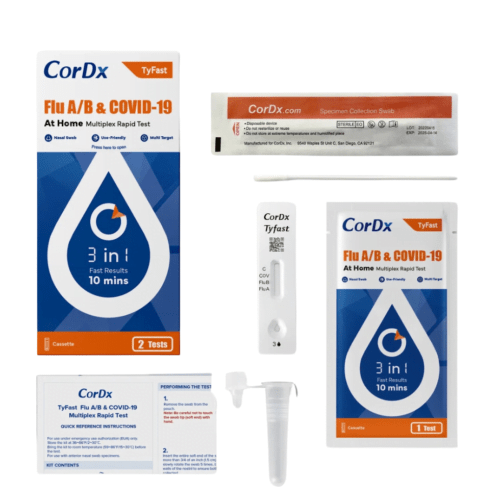
Flu A/B & COVID-19 Multiplex Rapid Test (3-in-1 Home Self-Test)
$14.99 – $21.99
 FDA EUA Approved (No. EUA240006)
FDA EUA Approved (No. EUA240006)
This test is authorized for the qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B protein antigens. This test is authorized for non-prescription home use with self-collected anterior nasal swab specimens from individuals aged 14 years or older, or with adult-collected anterior nasal swab specimens from individuals two (2) years or older.
Stay ahead of the curve and ensure your health with our latest Tyfast Flu A/B & COVID-19 Multiplex Rapid Home Test Kit (OTC options). This state-of-the-art rapid test empowers you to quickly check for both flu (A & B) and COVID-19 infection from the comfort of your home in just 10 minutes. Perfect for anyone experiencing symptoms of respiratory diseases, our kit offers peace of mind with swift and accurate results.
- Enjoy Fast Results: Get clear answers fast, enabling you to take the next steps sooner.
- Multi Target: Taking control of your health has never been easier or more convenient. Get fast, accurate, and reliable results, and stay one step ahead of flu and COVID-19.
- Rely on Precision: Trust in the clinical accuracy that accurately detects flu and COVID-19 antigens, giving you the certainty you need.
- User-Friendly: Our simple, straightforward design makes at-home testing a breeze, no matter where you are.
- Nasal Swab
Designed for efficiency and reliability, our Fluorescence Immunoassay Analyzer (FIA) system leverages advanced fluorescence detection technology to deliver accurate, high-quality test results with unparalleled speed.
Ideal for a broad range of applications, including diagnosing infectious diseases, cardiovascular conditions, diabetes, and more, this compact, fully automated benchtop analyzer is a powerhouse of functionality. It supports a wide variety of tests with its specialty menu, available in single-test ready-to-use formats for on-demand results. The intuitive interface ensures ease of use, while the robust system design promises minimal maintenance and continuous operation with a Mean Time Between Failures (MTBF) of over 370 days.
Enhance your laboratory’s productivity with our FIA’s full traceability and automation capabilities. Its bidirectional connectivity to both internal and external networks facilitates a seamless workflow. With features such as automated calibration, on-board dilution, and a comprehensive quality control management system, it reduces hands-on time and ensures the integrity of each test conducted.
Fast adoption is guaranteed thanks to the analyzer’s streamlined test loading process and minimal training requirements, making it an essential addition to any lab seeking to improve its diagnostic capabilities while ensuring accuracy and repeatability in test results.
INTENDED FOR: (But not limited to the list)
- Infectious Diseases:
COIVID-19 Rapid Test - Inflammatory Markers:
C-Reaction Protein(CRP),
Serum Amyloid A(SAA),
High-sensitivity C-reactive protein(hs-CRP)/C-Reaction Protein(CRP) - Diabetes:
Glycosylated Hemoglobin(HbA1C),
D-Dimer(DD),
Cystatin C(Cys-C),
Homocysteine(HCY),
Microalbumin(mAlb) - Tumor Screening:
Carcinoembryonic Antigen(CEA) - Cardiac Marker:
Cardiac Troponin I(cTnI) - Reproduction / Fertility:
Follicle-Stimulating Hormone(FSH),
Luteinizing Hormone(LH),
Human Chorionic Gonadotropin(HCG) - Hormone:
25-Hydroxyvitamin D(25-OH-VD)
Freeze Block
$99.99
Holds most 96-well plates
- Anodized aluminum Freeze Blocks maintain temperature at 0 degrees C for one hour after freezing and provide a stable platform for PCR reaction set-up.
- Pictured with the UltraPlate (#26190), sold separately.
-
Certified PCR Inhibitor FreeCertified Human DNA FreeCertified Pyrogen Free

The BeingBio RealTime High-Risk HPV kit is suitable for in vitro qualitative detection of 14 high-risk types of human papillomavirus (including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 66) nucleic acid material DNA in female cervical cell samples, and genotyping detection of HPV16 and HPV18 among them.

BeingBio Multiplex Real Time PCR Kit for HPV High-Risk Gene-type Screen kit is suitable for the detection of human papillomavirus (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) DNA typing in female cervical exfoliated cells. The kit is used for the auxiliary diagnosis of human papillomavirus infection, and can identify the specific types of human papillomavirus in each group. The test results of the kit are for clinical reference only and should not be used as the sole basis for diagnosis or exclusion.

BeingBio Freeze-Dried Multiplex Real Time PCR Kit for Mycoplasma hominis / Mycoplasma genitalium kit was used for qualitative detection of Mycoplasma hominis (MH) and Mycoplasma genitalium (MG) nucleic acid reproductive tract secretions samples. It can be used for aided diagnosis of MH and MG.