Desalting Gravity Flow Columns
$79.99 – $179.99Price range: $79.99 through $179.99
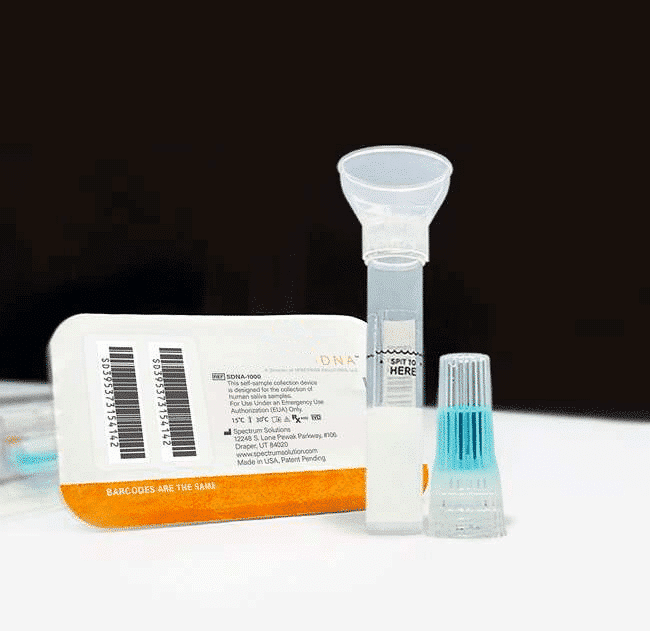
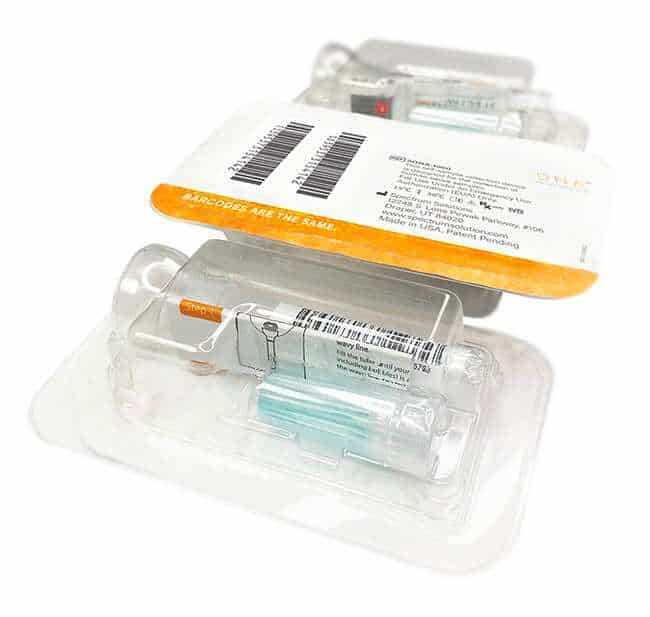
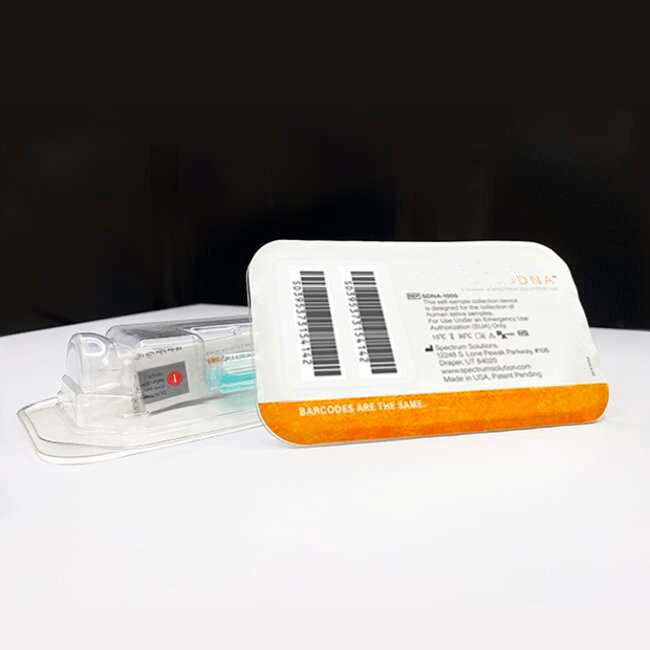
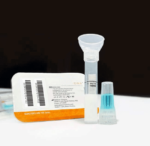
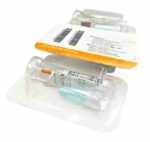
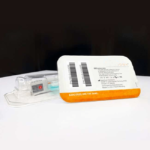
Whole Saliva Collection Kit

INTENDED USE:
The saliva collection system is designed for self-contained saliva sample collection which provides critical sample consistency while amplifying, preserving, and protecting transcripts at the point of collection for highly sensitive and highly specific analysis. Sample self-collection using a saliva collection kit provides 100% in-device live viral neutralization in 10 seconds at room temperature, mitigating any risk of exposure for downstream processes. The saliva kits have no special storage or transport temperature-control requirements for collected saliva samples and eliminate the need for UN3373 biohazard shipping designation with clearance from USPS, FedEx, & UPS.
FEATURES:
- First FDA Authorized COVID-19 saliva testing solution
- First FDA Authorized at-home saliva collection device for COVID-19 testing.
- Identify infections at their earliest stage before the onset of symptoms.
- In-device 100% inactivation of the live virus within the device at ambient temps
- Maintains critical bio-sample consistency.
- 99.998% testing accuracy & more sensitive
- No temperature-controlled storage or sample transport
- Post-collection stability with no degradation in sample efficacy
- Process multiple tests from a single collected sample
- Eliminate the need for UN3373 shipping designation.
- Supported system and validated testing process.
- At-home and direct-to-patient fulfillment
- simplicity and effectiveness of saliva collection devices have delivered a new standard of care for patients and laboratories.
SKU:
SDNA-1000 / SDNA-2000 / SDNA-3000 Rx only
Categories: Clinical Diagnostics, Specimens Collection
Description
ORDER INFORMATION:
| Cat. # | Description | Qty. |
|---|---|---|
| SDNA-1000 | Saliva Collection Device for FDA 510k Cleared DNA & RNA molecular diagnostic testing, specimen collection and extended analyte preservation at ambient temperatures. | 1/kit |
|
SDNA-2000 |
Saliva Collection Device, for DNA & RNA molecular diagnostic testing. CE mark approved and authorized. |
1/kit |
|
SDNA-3000 (Rx Only) |
Saliva Collection Device, for Rx Only – DNA & RNA molecular diagnostic testing. CE mark approved and authorized. |
1/kit |
SPECIFICATIONS:
Simplify the collection of saliva for commercial bioinformatic DNA testing applications, academic, research, biobanking, and as a bio-sample collection option for viral DNA/RNA COVID-19 testing for hospital networks, health departments and clinical research organizations with Spectrum™ Solutions SDNA Whole Saliva Collection Device.
- Single device for DNA/viral RNA bio sample collection, transportation, & storage.
- Intuitive 3-step design, proven to reduce customer collection errors.
- System maintains bio sample consistency.
- Significantly reduces costs associated with sample failures & recollection.
- Tube compatible with automated platforms.
- Innovative, patented chemistry that preserves & protects both DNA/viral RNA transcripts, manages bacteria & mitigates any risk of infection throughout the testing process.
APPLICATIONS:
SDNA-1000 Saliva Collection Device —-FDA 510k Cleared DNA & RNA Molecular Diagnostic Testing
- [2023] FDA Class II Medical Device Clearance
- [2020] The FDA has granted special authorization (EUA202432) and device use for COVID-19 testing beyond research-only applications to additionally include clinical diagnostics. COVID-19 saliva-based diagnostic testing
SDNA-2000 Saliva Collection Device—-DNA & RNA Molecular Diagnostic Testing
- Specimen Collection and Extended Analyte Preservation at Ambient Temperatures
- CE Approved and authorized
- Health Canada Approved and authorized for COVID-19 testing
- Unsupervised adult sample self-collection
SDNA-3000 (Rx Only) Saliva Collection Device—-Rx Only – DNA & RNA Molecular Diagnostic Testing
- Specimen Collection and Extended Analyte Preservation at Ambient Temperatures
- CE Approved and authorized.
- Health Canada Approved and authorized for COVID-19 testing.
- Unsupervised adult sample self-collection
Additional information
| Product No |
SDNA-1000 ,SDNA-2000 ,SDNA-3000 Rx only |
|---|
Shipping & Delivery
Related products
Accu News® (LH) One-Step Ovulation Test
$2.99 – $179.99Price range: $2.99 through $179.99
Select options
This product has multiple variants. The options may be chosen on the product page
Methamphetamine (MET) Drugs Test
$59.99 – $109.99Price range: $59.99 through $109.99
Select options
This product has multiple variants. The options may be chosen on the product page