Human Papilloma Virus (HPV) Antigen Rapid Test Kit
Intended Use
This rapid visual immunoassay developed for the qualitative presumptive detection of HPV 16/18 E6 and E7 oncoproteins in cervical swab specimens from women. It is designed to support the diagnosis of cervical pre-cancer and cancer, offering a valuable ability particularly in low-resource settings with limited access to comprehensive screening methods. By detecting the critical oncoproteins responsible for cervical cell transformation, this test provides a simple, efficient, and cost-effective approach to evaluating HPV oncogenic activity.
SKU:
BB-BG-HPV 01
Categories: Cancer, Clinical Diagnostics, Infectious Diseases, Women's Health
Description
Key Benefits
The HPV Antigen Rapid Test Kit is designed for the qualitative detection of high-risk HPV types, particularly HPV 16 and 18, in cervical swab samples.
-
Early Detection: The test enables healthcare providers to identify women at risk of cervical cancer before the disease develops, allowing for timely intervention.
-
Rapid Results: Results can be obtained within 15 minutes, facilitating quick decision-making for patient care.
-
High Performance: These tests offer better sensitivity (>90%) for detecting cervical high-grade lesions compared to traditional screening methods.
-
Ease of Use: The test can be performed with minimal laboratory equipment, making it suitable for use in various healthcare settings.
-
Improved Screening Coverage: By providing a simple and accessible testing option, these kits can help increase screening coverage, especially in low-resource regions.
-
Cost-Effective: The rapid test format offers a more economical approach to cervical cancer screening compared to more complex laboratory-based methods.
-
Empowerment: These tests allow for self-sampling, empowering women to take control of their health, particularly in areas with limited healthcare access.
-
Bridging Health Disparities: The availability of these tests can help overcome regional health disparities by bringing essential cervical cancer prevention tools to underserved areas.
Specifications
| Test Item | Sample | Storage | Shelf-life |
| HPV | Female cervical swab | 2 – 30°C | 18 months |
Clinical Performance
| Parameter | LoD | PPA | NPA |
| HPV 16/18 E6 and E7 oncoproteins | 1.0 x 105 Copies/ml | 87.39% | 91.54% |
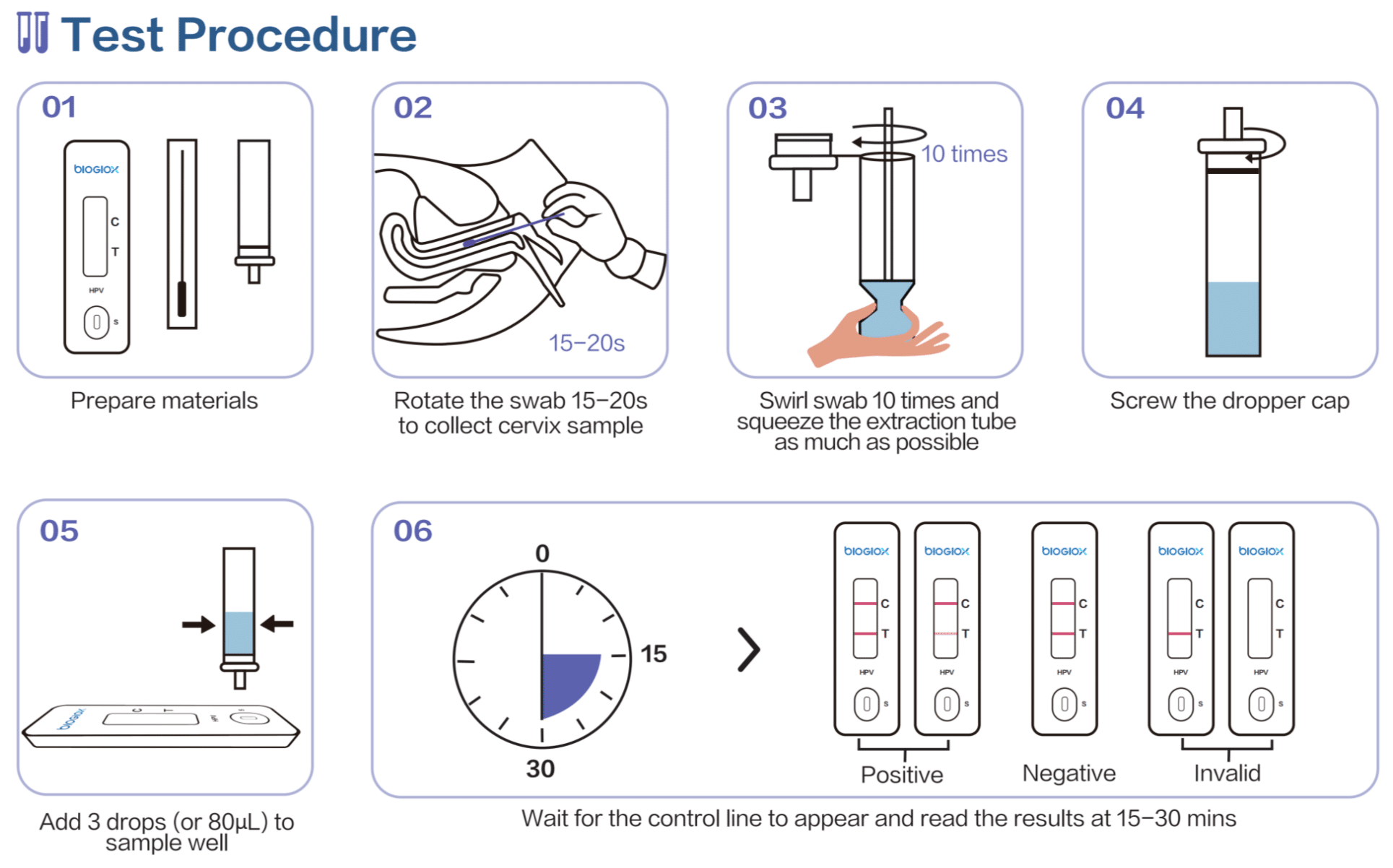
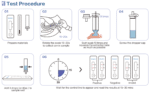
Instruction For Use
Additional information
| Product No |
BB-BG-HPV 01 |
|---|---|
| Quantity |
1 Test/box |
Shipping & Delivery
Related products
Cocaine (COC) Drugs Test – [FDA 510(k) Approved]
$25.99 – $109.99
Select options
This product has multiple variants. The options may be chosen on the product page
Ecstasy (MDMA) Drugs Test – [FDA 510(k) Approved]
$59.99 – $109.99
Select options
This product has multiple variants. The options may be chosen on the product page
Marijuana (THC) Drugs Test – [FDA 510(k) Approved]
$25.99 – $109.99
Select options
This product has multiple variants. The options may be chosen on the product page
Multi-Drugs Screen Test -(FDA 510(k) Approved)
$5.99 – $269.99
Select options
This product has multiple variants. The options may be chosen on the product page