Flu A/B & COVID-19 Multiplex Rapid Test (3-in-1 Home Self-Test)

This test is authorized for the qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B protein antigens. This test is authorized for non-prescription home use with self-collected anterior nasal swab specimens from individuals aged 14 years or older, or with adult-collected anterior nasal swab specimens from individuals two (2) years or older.
Stay ahead of the curve and ensure your health with our latest Tyfast Flu A/B & COVID-19 Multiplex Rapid Home Test Kit (OTC options). This state-of-the-art rapid test empowers you to quickly check for both flu (A & B) and COVID-19 infection from the comfort of your home in just 10 minutes. Perfect for anyone experiencing symptoms of respiratory diseases, our kit offers peace of mind with swift and accurate results.
- Enjoy Fast Results: Get clear answers fast, enabling you to take the next steps sooner.
- Multi Target: Taking control of your health has never been easier or more convenient. Get fast, accurate, and reliable results, and stay one step ahead of flu and COVID-19.
- Rely on Precision: Trust in the clinical accuracy that accurately detects flu and COVID-19 antigens, giving you the certainty you need.
- User-Friendly: Our simple, straightforward design makes at-home testing a breeze, no matter where you are.
- Nasal Swab
SKU:
CD-CO292-1 / CD-CO292-2
Categories: Clinical Diagnostics, COVID-19, Flu & RSV, Infectious Diseases
Description
The Influenza A/B & COVID-19 Multiplex Rapid Test (3-in-1 Home Self-Test) differentiates between Flu A/B with SARS-Cov-2.
Results are for the identification of Flu A/B and SARS-CoV nucleocapsid protein antigen, which is generally detectable in anterior nasal (nares) swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of the disease. An individual who tests positive with the Flu A/B and COVID-19 Ag Rapid Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
All negative results are presumptive and confirmation with a molecular assay, if necessary for patient management, may be performed. Negative results do not rule out Flu A/B or SARS-CoV infection and should not be used as the sole basis for treatment or patient management decisions, including infection control measures such as isolating from others and wearing masks. Negative results should be considered in in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with Flu and COVID-19.
Individual shall provide all results obtained with this product to their healthcare provider for public health reporting and to receive appropriate medical care.
TEST PROCEDURES
Step 1: Sample Collection
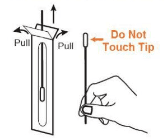
• Remove the swab from the pouch (Note: Be careful not to touch the swab tip (soft end) with fingers.)
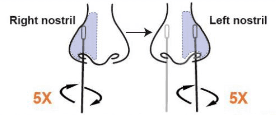
• Carefully insert the swab tip into one nostril about ½ to ¾ inch. Firmly and slowly rotate the swab 5 times, brushing against the inside walls of the nostril to ensure both mucus and cells are collected. (Note: 1. Do not push the swab further if you meet resistance. 2. For young children, do not insert more than ½ inch.)
• Using the same swab, repeat this process for the other nostril to ensure an adequate sample is collected from both nostrils. (Note: Failure to swab properly may cause incorrect results.)
Step 2: Sample Processing
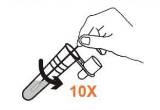
• Insert the swab in tube to the bottom.
• Rotate the swab at least 10 times while pressing the swab head against the bottom and side of the tube.
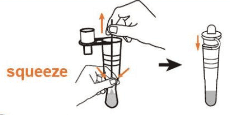
• Remove the swab while squeezing the sides of the tube to express as much liquid as possible from the swab.
• Attach the dropper tip firmly onto the tube.
Step 3: Sample Testing
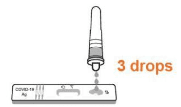
• Gently squeeze the tube and dispense 3 drops of solution into the sample well. (Note: A false negative or invalid result may occur if less than 3 drops of solution are added to the Sample well.)
Step 4: Testing Result
• The result is valid when read at 10 – 30 minutes. If a POSITIVE result is obtained within 10 minutes, the result should also be considered valid.

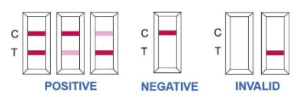
Step 5: Guidelines on Test Result Interpretation
Shipping & Delivery
Related products
Accu News® (LH) One-Step Ovulation Test
$2.99 – $179.99Price range: $2.99 through $179.99
Select options
This product has multiple variants. The options may be chosen on the product page
Marijuana (THC) Drugs Test
$25.99 – $109.99Price range: $25.99 through $109.99
Select options
This product has multiple variants. The options may be chosen on the product page
Morphine (MOP) Drugs Test
$59.99 – $109.99Price range: $59.99 through $109.99
Select options
This product has multiple variants. The options may be chosen on the product page