SARS-CoV-2/Flu A/FLu B/RSV/ADV/MP Antigen Rapid Test Kits
Intended Use
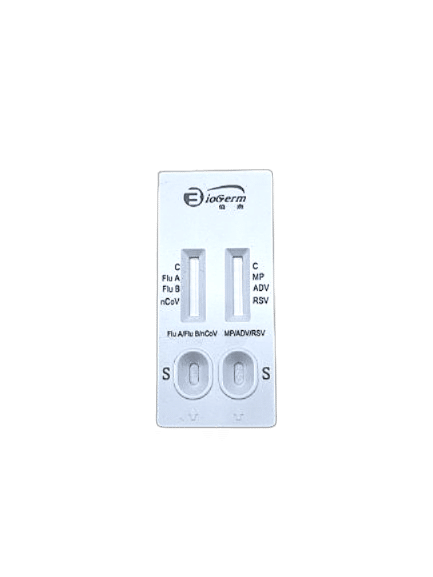
The visual reading test kit is a rapid, qualitative diagnostic tool designed for the simultaneous detection of antigens from six major respiratory pathogens: SARS-CoV-2, Influenza A, Influenza B, Respiratory Syncytial Virus (RSV), Adenovirus, and Mycoplasma pneumoniae. This test is intended for use with throat swab specimens collected from individuals exhibiting symptoms of respiratory tract infections, such as cough, fever, sore throat, or difficulty breathing.
The primary purpose of this six-in-one respiratory panel is to provide accurate and timely identification of the causative pathogen responsible for respiratory illnesses. By detecting specific antigens associated with these pathogens, the kit aids in differentiating between viral and bacterial infections, enabling healthcare providers to make informed decisions regarding treatment and infection control measures.
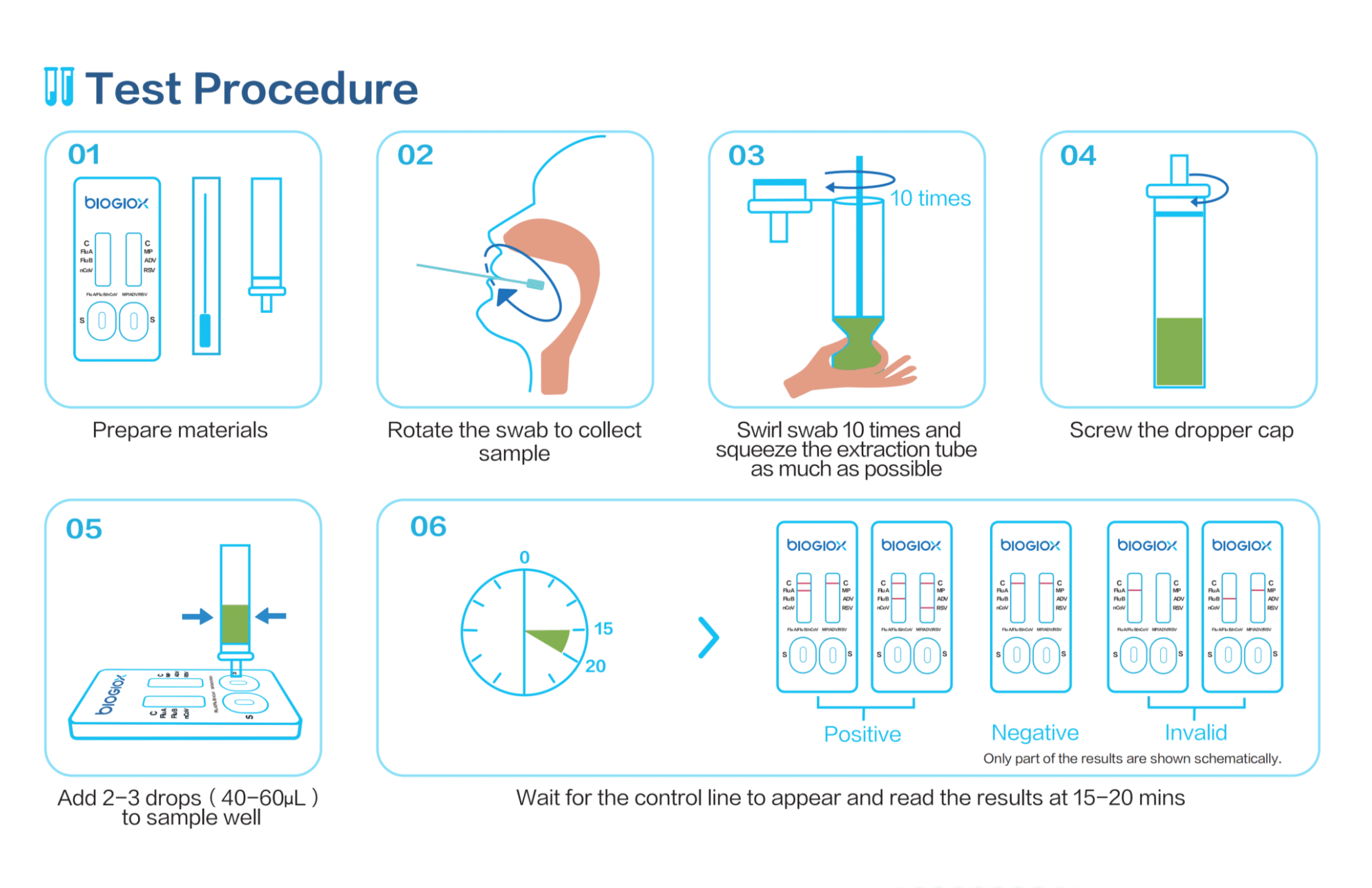
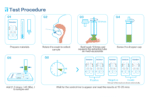
This test is particularly valuable in clinical settings, such as hospitals, outpatient clinics, and emergency departments, where rapid diagnosis is critical for patient management and preventing the spread of infectious diseases. It is also suitable for use in point-of-care (POC) settings, such as your doctor office or at-home testing, offering results within 15–20 minutes without the need for specialized laboratory equipment.
SKU:
BB-INF61-01
Categories: Clinical Diagnostics, COVID-19, Flu & RSV, Infectious Diseases
Description
Specification
| Test Item | Sample | Storage | Shelf-life |
| SARS-CoV-2/Flu A/Flu B/RSV/ADV/MP | Throat swab | 2 – 30°C | 18 months |
Clinical Performance
| Parameter | LoD | PPA | NPA | Percent Agreement |
| Flu A (H3N2) | 1.22 x 10 TCID50 /ml | 98.15% | 99.65% | 99.52% |
| Flu A (H1N1) | 3.25 x 10 TCID50 /ml | |||
| Flu A /2000 (H1N1) | 1.25 TCID50 /ml | |||
| Flu B /Victoria | 1.0 x 102 TCID50 /ml | 97.62% | 99.32% | 99.20% |
| Flu B /Yamagata | 5.25 x 102 TCID50 /ml | |||
| SARS-CoV-2 | 2.0 x 102TCID50 /ml | 97.88% | 99.41% | 99.12% |
| MP | 7.10 x 103 TCID50 /ml | 97.39% | 99.47% | 99.28% |
| ADV | 6.0 x 103 TCID50 /ml | 97.80% | 99.91% | 99.76% |
| RSV | 1.2 x 103 TCID50 /ml | 97.37% | 96.28% | 99.32% |
Key Features and Benefits
- Comprehensive Detection: Simultaneously identifies six common respiratory pathogens, reducing the need for multiple tests and streamlining the diagnostic process.
- Rapid Results: Provides results in as little as 15–20 minutes, enabling timely clinical decisions.
- Ease of Use: Visual readout eliminates the need for complex instrumentation, making it accessible for use in various healthcare settings, including resource-limited environments.
- High Sensitivity and Specificity: Designed to minimize false positives and negatives, ensuring reliable results.
- Non-Invasive Sampling: Utilizes throat swabs, a convenient and widely accepted method for respiratory specimen collection.
Clinical Applications
- Differential Diagnosis: Distinguishes between viral and bacterial respiratory infections, aiding in appropriate antibiotic stewardship.
- Pandemic and Seasonal Outbreak Management: Facilitates the rapid identification of SARS-CoV-2, Influenza A/B, and RSV during peak respiratory illness seasons.
- Infection Control: Helps prevent the spread of contagious respiratory pathogens in healthcare and community settings.
- Point-of-Care Testing: Ideal for use in urgent care, outpatient clinics, and remote healthcare facilities.
Additional information
| Product No |
BB-INF61-01 |
|---|
Shipping & Delivery
Related products
100 ml Reagent Reservoir
$199.99
Select options
This product has multiple variants. The options may be chosen on the product page
Amphetamine (AMP) Drugs Test
$59.99 – $109.99Price range: $59.99 through $109.99
Select options
This product has multiple variants. The options may be chosen on the product page
DeepWell Plates
$189.99
Select options
This product has multiple variants. The options may be chosen on the product page